Introduction
Up-regulation of inhibitory receptors, such as programmed death-1 (PD-1), is an integral component of acute myeloid leukemia (AML) immune evasion, chemotherapy resistance, and disease progression. Early phase clinical trials investigating immune checkpoint inhibitors (ICIs) in patients with AML have demonstrated promising findings. We previously reported that pembrolizumab administration on day 14 after high-dose cytarabine (HiDAC) salvage chemotherapy in patients with relapsed/refractory (R/R) AML was safe, feasible, and associated with encouraging clinical outcomes. PD-1 blockade prior to allogeneic stem cell transplant (alloSCT) has been associated with increased risks of alloSCT-related toxicity in lymphoma. AlloSCT remains the most established treatment paradigm for curative intent in AML patients who achieve a complete response (CR); however, there are limited data on the clinical outcomes and safety of ICI prior to alloSCT in AML. We set out to compare clinical outcomes of AML patients receiving pembrolizumab prior to alloSCT versus a control group of AML patients receiving alloSCT without prior ICI exposure.
Methods
We assessed clinical outcomes of patients who enrolled on a phase II clinical trial of HiDAC followed by pembrolizumab 200 mg IV on day 14 (NCT02768792) for R/R AML and subsequently underwent an alloSCT. Date of alloSCT was required to be >30 days from last dose of pembrolizumab. We matched each trial participant who underwent an alloSCT in a 1:2 ratio with controls who received alloSCT at our institution since 2016. Patients were matched on age, sex, age-adjusted hematopoietic cell transplantation-comorbidity index (HCT-CI), donor type, and conditioning intensity. The nonparametric Jonckheere-Terpstra test was used to test for a difference in the ordered severity categories of acute graft versus host disease (aGVHD) within 100 days of transplant. The time-to-event estimates for overall survival (OS) and relapse-free survival (RFS, event is either death or relapse) were calculated using the Kaplan-Meier method and compared using a log rank test.
Results
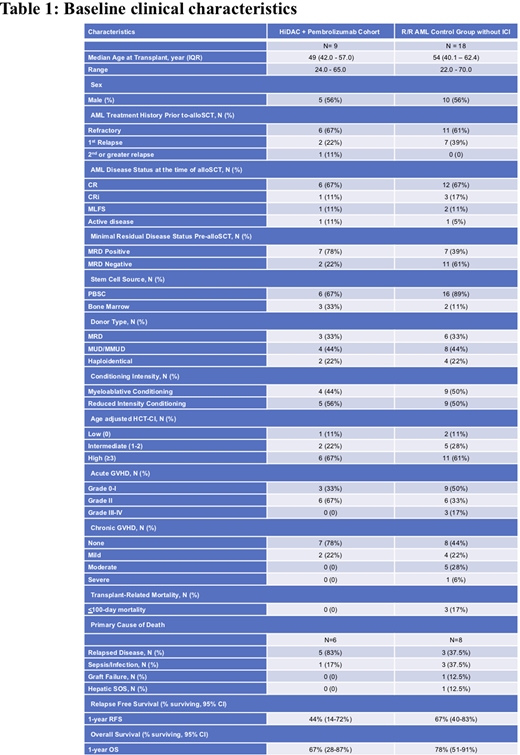
Nine out of 37 (24.3%) R/R AML patients treated with HiDAC followed by pembrolizumab received an alloSCT. Baseline characteristics of the R/R AML patients who received pembrolizumab versus R/R AML controls who underwent alloSCT are described in Table 1. One patient in each cohort received an alloSCT with evidence of active disease by flow cytometry (>5% blasts) or hematopathology IHC evaluation, whereas all other patients in both arms were in CR/CRi or MLFS at the time of alloSCT.
The pattern of aGVHD severity was similar with the exception of 3 control patients (3/18 or 17%) having grade III-IV aGVHD versus 0/9 pembrolizumab patients (p = 0.92). Notably, 7/9 (78%) patients in the pembrolizumab cohort had no evidence of chronic GVHD.
There was a nonsignificant increase in relapses (6/9: 67% vs. 6/18: 33%; p = 0.22) and deaths (6/9: 67% vs. 8/18: 44%, p = 0.42) in pembrolizumab versus control patients, respectively. The median follow-up for survivors was 23 months. Median OS was 21 months for pembrolizumab cohort versus 25 months for control patients. One-year OS was 67% (95% CI, 28%-87%) for pembrolizumab patients versus 78% (95% CI, 51%-91%) for control patients, p = 0.34. One-year RFS was 44% (95% CI, 14%-72%) for pembrolizumab patients versus 67% (95% CI, 40%-83%) for control patients, p = 0.14.
Conclusion
Treatment with ICI represents a promising therapeutic strategy for AML. Among a cohort of patients treated with HiDAC followed by pembrolizumab for R/R AML, alloSCT appeared to be feasible without increased risk of GVHD or early mortality. Although a small number of patients received alloSCT after pembrolizumab, OS appeared comparable to R/R AML controls who did not receive ICI. The increase in relapse rates seen in the pembrolizumab cohort may have been impacted by the higher proportion of patients with measurable residual disease (MRD) prior to alloSCT (78% vs. 39%, respectively). These data warrant further investigation of ICI prior to and after alloSCT in high-risk AML patients.
Tschernia:AstraZeneca: Research Funding. Vincent:GeneCentric Therapeutics: Consultancy. Coombs:Abbvie: Consultancy, Honoraria; Genentech: Honoraria; AstraZeneca: Honoraria; MEI Pharma: Honoraria; LOXO Oncology: Honoraria; Octapharma: Honoraria; Novartis: Honoraria. DeZern:MEI: Consultancy; Celgene: Consultancy, Honoraria; Abbvie: Consultancy; Astex: Research Funding. Luznik:WindMil Therapeutics: Patents & Royalties: Patent holder; Genentech: Research Funding; AbbVie: Consultancy; Merck: Research Funding, Speakers Bureau. Riches:Biointelect: Consultancy. Gojo:BMS: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Merck: Research Funding; Amphivena: Research Funding; Genentech: Research Funding. Zeidner:AbbVie: Honoraria, Other: Independent Review Committee; Agios: Honoraria; Daiichi Sankyo: Honoraria; Genentech: Honoraria; Pfizer: Honoraria; Takeda: Consultancy, Honoraria, Other: Travel Reimbursement, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AsystBio Laboratories: Consultancy; AROG: Research Funding; Forty-Seven: Other: Travel Reimbursement, Research Funding; Merck: Research Funding; Sumitomo Dainippon Pharma Oncology, Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal